Bioreactors: A Comprehensive Guide to Its Capabilities and Applications
.png)
Bioreactors are vessels used to carry out a variety of biological processes involving organisms such as microorganisms and animal/plant cells under controlled conditions (temperature, pH, oxygen, nutrient levels etc.). With capabilities to precisely control key parameters, bioreactors enable optimal growth conditions leading to high productivity.
Over the past few decades, bioreactors have become indispensable tools in a wide range of industries from pharmaceuticals, biofuels and foods to chemicals, wastewater treatment and beyond.
This article provides a comprehensive guide to bioreactor systems, their capabilities, components, types, key applications and growth opportunities. We also highlight how IT Tech’s portfolio of products and services can help you set up and optimize your bioprocesses using state-of-the-art bioreactors.
What are Bioreactors and Their Key Capabilities?
A bioreactor refers to any manufactured or engineered device that supports an optimal environment for biological processes involving organisms to generate desired metabolic products efficiently on a large scale.

Key Capabilities and Features
- Precise process control and automation - Key parameters like temperature, pH, DO, foam/level and nutrient feed can be precisely monitored and controlled to maintain optimized conditions for enhanced productivity.
- Aseptic environment - Bioreactors enable sterile culture conditions critical for growth of microbes and cells.
- Homogenous mixing and mass transfer - Built-in impellers enable uniform mixing and oxygen transfer ensuring uniform growth across the vessel.
- High throughput - Bioreactors are scalable to volumes from bench scale testing (100 mL - 10 L) up to industrial-scale 1000+ cubic meter capacities, enabling high volume throughput.
- Flexibility - Bioreactors can be customized to meet process-specific needs in terms of material of construction, type of agitation, sensors, probes etc.
These features make bioreactors indispensable for efficient, large-scale bio-based processes across industries.
Key Components of a Bioreactor System
A bioreactor system comprises several components for maintaining optimized culture conditions and allowing seamless process execution. While configurations can vary based on scale and mode of operation, below we explore some major components common across setups:
1. Vessel
The vessel forms the heart of a bioreactor system. Usually cylindrical or slightly conical in shape for uniform mixing, vessels can range from bench scale 100 mL to large cubic meter sizes based on process needs. They are designed for steam sterilization or single-use pre-sterilized models are available too. Different materials like stainless steel or specialty steel alloys are chosen based on durability, cleanability, corrosion resistance and meeting verification needs. Location and number of ports & openings are also customized based on process needs.
2. Agitation assembly
The stirring system introduces homogeneity within the vessel environment ensuring uniform nutrient distribution and oxygen transfer besides preventing cell/particle sedimentation. Bench scale vessels use magnetic drives while pilot/industrial units use top entry or side mounted mechanical agitators. Their rotation speed, size and type is optimized to generate optimal mixing with minimal shear. Sparge rings may also supplement mechanical assembly for additional air/oxygen transfer.
3. Aeration module
For aerobic fermentations, the aeration system ensures optimal dissolved oxygen levels by sparging sterile air/oxygen. This also strips out CO2 preventing inhibition. The rate of aeration can be automatically varied based on dissolved oxygen readings via feedback control. While small vessels rely on surface aeration alone, larger units require additional specialized spargers.
4. Control units
Automated bioreactors require sensors to relay critical process parameters to external control hardware like PLCs or industrial PCs which run PID control algorithms to keep variables like temperature, pH and DO within desired range by actuating pumps, valves or heating elements. They ensure minimal manual intervention with options for remote monitoring/control via networks.
5. Probes, sensors & transmitters
Key parameters like pH, DO and temperature within the bioreactor are continuously tracked using specialized autoclavable electrochemical probes containing electrolytes which generate electrical signals proportional to concentration of species. Paired temperature sensors also compensate readings for accurate results. Transmitters receive this data and convert signals into readable digital values before routing to controller.
6. Tubing, fittings & pipes
Bioreactor tanks require inlet and outlet streams typically via piping for transferring sterile media, inoculum, acids/bases, antifoams etc. Peristaltic pumps help movement fluids through tubing lines which are chosen for biocompatibility. Tri-clamp & aseptic fittings meeting bio-pharma grade requirements provide secure leak-proof connections.
7. Ports & openings
Vessels contain multiple ports & specialized closures to introduce probes, sensors, spargers, sample collection, inlet/outlet lines for adding media. These opening also facilitate the cleaning & sterilization steps. Their design ensures integrity against leaks/contamination. Steam in/condensate out ports also serve for SIP/CIP needs.
8. Foam control
Many bioprocesses unfortunately also generate excessive foam during cell growth or gas sparging steps. Foam can be detrimental by increasing fluid pressure, blocking sensors or contaminating outlet gas filters. Hence most reactors have specialized ports for controlled delivery of sterile antifoaming agents like silicone oils to mitigate foam. Level sensors gauge foam and trigger agent delivery via pumps. The amount, timing and type of antifoam is optimized for each process.
9. Platform/skid & motor
A structural skid mounted platform houses larger bench and pilot scale bioreactor set-ups keeping them stationary for safe operation. It bears the load of heavy vessels and contains integrated control panel housing instrumentation and electrics besides air compressor & steam generator if needed. Motors specifically designed for sterile environments drive agitators.
10. Software interface
A digital software platform offers a unified window for operators to visualize all parameters, monitor trends and if needed program/change control recipes or manual overrides for managing the entire process via machine-human interface. Large setups also enable remote operation. Data recording for batch traceability and regulatory needs is also possible.
Major Types of Bioreactor Configurations
Bioreactors come in different configurations based on type of biological process, scale etc. Some key varieties include:
1. Stirred-tank
Most common type. Contains an agitator for mixing and suspension of solids. Suitable for microbial/animal/plant cell cultures. Different types of impellers provide optimal mixing.

Application - Commonly used in bio-pharma processes for growth of bacteria/yeast to produce antibiotics, enzymes, vitamins etc. Also used for propagation of plant or animal cells and tissues.
2. Airlift
Contains a gas sparger to circulate air/gas through culture media enabling circulation and mixing.
Benefits - No moving parts, simple design
Application - Used for plant/algae/photo bioreactor cultures due to excellent gas-liquid oxygen transfer
3. Packed Bed Reactor (PBR)
Contains inert porous substrate material on which biofilm grows. Media fed from top flows through and gets reacted.

Application - Wastewater treatment, biochemical, fermentation processes.
4. Membrane Bioreactor
Combines activated sludge process with membrane filtration like microfiltration/ultrafiltration for simultaneous reaction and separation.
Benefits - Enables very high cell density cultures; excellent for continuous processing of labile products.
Application - Continuous beer brewing, wines, pharmaceutical by-products recovery.
5. Photo BioReactor (PBR)
Specially designed for growing light-dependent cells like microalgae and cyanobacteria using sunlight/artificial lighting. Made up of specialized translucent tubing/flat panels optimized to provide large illumination surface area.
Application – Microalgal culture for biofuels, nutraceuticals etc.
Key Application Areas and Major End-use Sectors
Thanks to their excellent process control, reproducibility and reliability for optimizing bioprocesses, bioreactors serve as indispensable production platforms across industries for commercial scale biological manufacturing.

Below we explore some major end-use sectors using bioreactors and their key application examples:
• Biopharmaceuticals
One of the largest application areas is within biopharma which involves microbial fermentation of bacterial/fungal strains or mammalian/stem cell cultivation to produce antibiotics, biologics, vaccines and drug metabolites using fed-batch & perfusion bioreactors. As per one estimate, over 50% of all approved pharmaceuticals involve a bioreactor step.
• Biofuels and Bioenergy
Bioreactors enable large scale algal cultivation to produce triglyceride-rich biomass as feedstock for biodiesel production. They also drive cellulosic ethanol production from lignocellulosic biomass using microbial fermentation. Photobioreactors also help produce biohydrogen.
• Food, Feed and Nutraceuticals
Bioreactors drive commercial production of GRAS-status supplements like amino acids, vitamins, organic acids, natural pigments and probiotics that serve as healthy functional ingredients for foods, nutraceuticals, animal feed and aquaculture industries.
• Wastewater Biotreatment
Specifically designed bioreactors with immobilized microbes on fixed biofilm surfaces serve in both aerobic (activated sludge process) and anaerobic digestion of organic pollutants enabling water recycling and waste-to-value conversion.
• Bioplastics and Biomaterials
Optimized bioreactors produce hydroxyalkanoate monomers that are polymerized to form novel biodegradable polyhydroxyalkanoates (PHA) bioplastics to replace conventional synthetics for packaging industry.
• Biosensors and Diagnostics
Microbial bioreactors cultures supply specific enzymes/proteins that serve as recognition elements in cutting edge biosensors for rapid diagnosis of diseases, hormone levels and infections.
Clearly, bioreactors form indispensable production assets enabling execution of diverse bioprocesses serving a vast spectrum of end-use application areas at commercial scales across sectors.
Market Outlook
With expanding interest in sustainable technologies globally to transition away from conventional fossil-fuel driven synthesis - markets relying on bioreactors for commercial scale biomanufacturing are projected for steep growth worldwide in coming years.
Valued at nearly US$ 16 billion currently, the bioreactors market is forecasted to expand at brisk CAGR of over 15% through 2026, as per one report by leading research firm Acumen.
Key growth factors include - expanding biopharma sector driving demand for new biologic drugs; growing consumer preference for bio-based chemicals, polymers and fuels given sustainability benefits and government emphasis on eco technologies via funding, legislation and subsidies to drive rapid adoption across developing and emerging nations.
How Can IT Tech Help Your Bioprocess Needs?
IT Tech offers an extensive range of fermenters, bioreactors and associated instrumentation supporting solutions from renowned global brands tailored to serve industries spanning pharmaceuticals, chemicals, food/feed, biodiesel, agro-based products, wastewater treatment and more.
Our product portfolio comprises bench-top lab and pilot scale bioreactor systems up to large-scale production platforms with specialized autoclavable/single-use sensors and process analytical tools enabling real-time bioprocess optimization.
We also offer customized bioreactor design, selection and configuration
Subscribe to our newsletter
Stay updated with IT-Tech Insights
Related posts
Check out other IT- Tech Scientific Resources
.png)
Integrating Bioreactors & Fermentors for Lab Efficiency
Discover expert tips and best practices for seamlessly integrating bioreactors and fermentors into your laboratory workflow. From selecting the right equipment to optimizing growth conditions, elevate your scientific endeavors with our comprehensive guide.
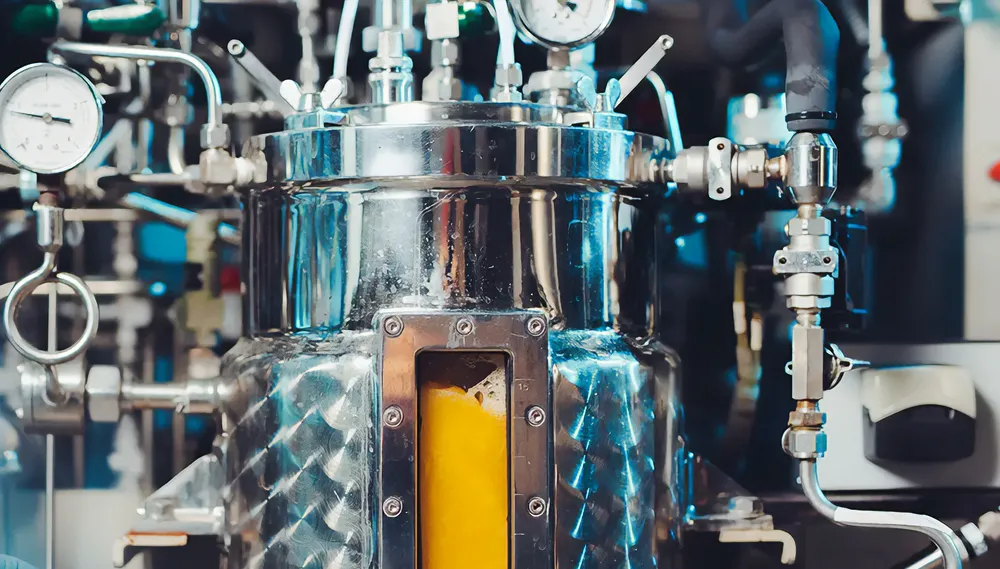
Transforming Scientific Discovery with Bioreactors and Fermentors
Explore the transformative potential of bioreactors and fermentors in driving cutting-edge research across biotechnology, pharmaceuticals, and more. Discover the latest advancements shaping the future of scientific innovation.
.png)
Making the Right Investment: A Detailed Analysis of Bioreactors
Discover the definitive guide to making informed investments in bioreactors. Explore the technical nuances, economic considerations, and supplier partnerships crucial for maximizing returns in bioprocessing. From single-use to stainless steel, gain insights to navigate the complexities and optimize your lab or production facility with expert advice and tailored solutions.

























































.png)








