Integrating Bioreactors and Fermentors into Your Workflow: Tips & Best Practices
.png)
In today's rapidly advancing scientific landscape, the integration of bioreactors and fermentors into laboratory workflows is essential for various research and production processes. Whether you're a lab technician, project manager, procurement manager, or C-suite executive in the scientific industry, understanding how to seamlessly incorporate these vital pieces of equipment is crucial for achieving optimal results. In this comprehensive guide, we'll delve into the intricacies of integrating bioreactors and fermentors into your workflow, providing you with valuable tips and best practices every step of the way.
Overview of Bioreactors and Fermentors

Source: Lab1st
Before delving into integration strategies, it's crucial to gain a comprehensive understanding of bioreactors and fermentors, including their distinct characteristics, functionalities, and applications.
Bioreactors
Bioreactors serve as versatile tools for cultivating cells, tissues, or organs in precisely controlled environments. These sophisticated systems offer researchers and scientists the ability to manipulate and optimize growth conditions with unparalleled precision. Key features of bioreactors include:
- Cell Culture: Bioreactors are commonly employed in various fields, including tissue engineering, regenerative medicine, and pharmaceutical research, to culture cells and tissues for experimental or therapeutic purposes.
- Precise Control: One of the primary advantages of bioreactors is their ability to regulate essential parameters such as temperature, pH, dissolved oxygen levels, and agitation speed. This precise control ensures optimal growth conditions for the cultured cells, enhancing experimental reproducibility and efficiency.
Applications
Bioreactors find applications in a diverse range of scientific disciplines, including but not limited to:
- Tissue Engineering: Cultivation of engineered tissues for regenerative medicine applications.
- Cell Therapy: Production of therapeutic cells for transplantation and cell-based therapies.
- Pharmaceutical Research: Screening and production of biologics, vaccines, and recombinant proteins.
Fermentors
Fermentors, while sharing some similarities with bioreactors, are specifically tailored for microbial or cell fermentation processes. These specialized systems are designed to support large-scale production of various products through fermentation, such as pharmaceuticals, biofuels, and food additives. Key features of fermentors include:
- Microbial Fermentation: Fermentors are primarily utilized for the cultivation of microorganisms, including bacteria, yeast, and algae, for the production of a wide range of bio-based products.
- Industrial Scale Production: Unlike bioreactors, which are often employed at smaller scales for research purposes, fermentors are commonly utilized in industrial settings for large-scale production. They offer robustness, scalability, and efficiency required for commercial production processes.
- Control Parameters: Similar to bioreactors, fermentors enable precise control over critical parameters such as temperature, pH, foam, and dissolved oxygen levels. This control ensures optimal growth conditions for the target microorganisms, resulting in high product yields and quality.
Applications
The applications of fermentors span across various industries, including:
- Pharmaceuticals: Production of therapeutic proteins, antibiotics, and vaccines through microbial fermentation.
- Biofuels: Cultivation of microorganisms for the production of bioethanol, biodiesel, and other renewable fuels.
- Food and Beverage: Fermentation of food additives, enzymes, and probiotics for food and beverage industries.
While bioreactors excel in cell culture applications for research and therapeutic purposes, fermentors are tailored for large-scale microbial fermentation processes in industrial settings. Understanding the distinctions between these two types of reactors is essential for selecting the appropriate equipment and designing effective integration strategies within laboratory workflows.
Choose Reactor Type Based on Application
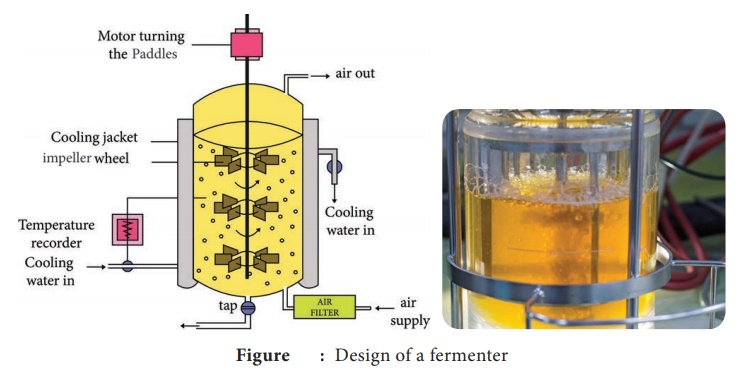
Source: BrainKart
Selecting the appropriate type of reactor is a crucial decision that significantly impacts the success of your experiments or production processes. Whether you opt for a bioreactor or fermentor depends on several factors, including:
Type of Organism
Consider the nature of the organism you intend to culture. Bioreactors are typically preferred for mammalian cell culture, tissue engineering, and cell therapy applications, whereas fermentors are better suited for microbial fermentation processes involving bacteria, yeast, or algae.
Scale of Production
Evaluate the scale at which you plan to operate. Bioreactors are commonly used in research and development settings or for small-scale production, whereas fermentors excel in large-scale industrial production due to their robustness and scalability.
Monitoring and Control Requirements
Assess the specific monitoring and control needs of your application. Bioreactors offer precise control over parameters such as temperature, pH, and dissolved oxygen levels, making them suitable for sensitive cell culture applications. Fermentors, meanwhile, are equipped to handle parameters relevant to microbial fermentation processes, such as foam control and agitation.
Consulting with equipment experts or suppliers can provide invaluable insights into selecting the optimal system for your needs, taking into account factors such as performance, scalability, and budget constraints.
Select Size and Features

Source: BIOTOP
Once you've determined the type of reactor required, carefully consider the size and features that align with your production requirements and budget constraints:
Size
Choose a reactor size that matches the scale of your operations. Whether you're operating on a benchtop scale or an industrial scale, select a vessel volume that can accommodate your desired production capacity without compromising efficiency or space constraints.
Essential Features
Prioritize features that enhance operational efficiency and data tracking capabilities. Look for reactors with automated control systems, robust data logging capabilities, and integrated monitoring systems to ensure seamless operation and accurate data acquisition.
Determine Placement in Lab

Source: ResearchGate
Proper placement of bioreactors and fermentors within your laboratory is critical for optimizing workflow efficiency and ensuring safety:
Space Considerations
Assess available space and layout considerations to determine the optimal location for your reactor setup. Ensure adequate clearance for equipment access, maintenance, and personnel movement to minimize disruptions and maximize efficiency.
Infrastructure Requirements
Evaluate power requirements, ventilation needs, and utility connections to support your reactor setup. Ensure sufficient electrical capacity, proper ventilation for heat dissipation, and appropriate utility connections for water, gas, and waste disposal.
Environmental Monitoring
Implement robust environmental monitoring systems to safeguard experiments and personnel against potential hazards. Monitor factors such as temperature, humidity, and air quality to maintain optimal conditions for reactor operation and personnel safety.
Train Personnel on Proper Use

Source: KGI
Effective utilization of bioreactors and fermentors requires comprehensive training for laboratory personnel:
Operation and Maintenance
Provide training on reactor operation, maintenance procedures, and safety protocols to ensure proper handling and care of equipment. Cover topics such as inoculation techniques, parameter control, cleaning procedures, and emergency response protocols.
Data Management
Educate personnel on data management practices, including data logging, interpretation, and analysis. Ensure proficiency in using software interfaces, troubleshooting common issues, and documenting experimental procedures for traceability and reproducibility.
Integrate with Upstream and Downstream Processes

Source: ResearchGate
Seamless integration of bioreactors and fermentors with upstream and downstream processes is essential for maintaining workflow continuity and maximizing productivity:
Streamlining Procedures
Optimize workflow efficiency by streamlining procedures for inoculum preparation, culture transfer, and harvest transfer. Coordinate with upstream and downstream operations to minimize bottlenecks and ensure smooth transition between process steps.
Supporting Equipment
Identify supporting equipment, such as peristaltic pumps or purification systems, needed to facilitate upstream and downstream processes. Ensure compatibility with reactor setup and consider automation options to streamline operations and minimize manual intervention.
Monitor and Optimize Conditions

Source: AtlasScientific
Continuous monitoring and optimization of growth conditions are essential for achieving reproducible results and maximizing product yields:
Define Setpoints
Establish optimal setpoints for critical parameters based on experimental requirements and process optimization studies. Monitor parameters such as temperature, pH, and dissolved oxygen levels to maintain optimal growth conditions throughout the culture process.
Real-time Monitoring
Utilize advanced monitoring systems to track growth kinetics, monitor culture viability, and detect deviations from setpoints in real-time. Implement automated feedback control systems to adjust parameters and maintain stability during dynamic process conditions.
Maintain Strict Aseptic Technique
Preventing contamination is paramount in bioreactor and fermentor operations to ensure the integrity of cultures and the reliability of results:
Sterile Procedures
Adhere to stringent aseptic techniques during reactor setup, operation, and sampling procedures. Implement proper sterilization protocols for equipment and media, and maintain a clean working environment to minimize the risk of contamination.
Personnel Training
Provide comprehensive training on sterile techniques, including aseptic handling, cleanroom protocols, and contamination prevention strategies. Emphasize the importance of maintaining sterile conditions throughout the experimental process to preserve culture integrity and ensure data validity.
Schedule Regular Cleaning and Calibration
Routine cleaning and calibration are essential for maintaining optimal performance and prolonging equipment lifespan:
Cleaning Procedures
Follow manufacturer guidelines for cleaning and sterilization procedures to ensure proper maintenance of equipment. Disassemble and clean reactor components regularly, and verify effectiveness using microbial testing and validation protocols.
Calibration
Calibrate sensors and probes regularly to ensure accuracy and reliability of data acquisition. Conduct routine calibration checks and performance verification tests to maintain consistency and traceability in experimental results.
Analyze Data to Refine Processes
Caption: Analyzing data helps refine processes and improve efficiency.
Alt text: A scientist analyzing data on a computer screen with graphs and charts related to bioreactor operations.
Source:
Data analysis is critical for optimizing bioreactor and fermentor operations and improving process efficiency:
Data Interpretation
Analyze data generated by reactor systems to identify trends, optimize process parameters, and troubleshoot issues. Utilize statistical analysis tools and visualization techniques to extract meaningful insights from experimental data and refine process strategies accordingly.
Process Optimization
Iteratively refine process parameters based on data-driven insights to enhance product quality, increase yields, and reduce production costs. Implement adaptive control strategies and feedback mechanisms to dynamically adjust process conditions and maintain optimal performance throughout the culture cycle.
Adopt Quality Standards
Adherence to stringent quality standards is essential for ensuring the reliability and consistency of bioreactor and fermentor operations:
Regulatory Compliance
Comply with relevant regulatory requirements and industry standards governing bioprocess manufacturing. Implement good manufacturing practices (GMP), establish standard operating procedures (SOPs), and conduct regular audits to ensure compliance and traceability in production processes.
Quality Assurance
Implement quality assurance protocols to validate equipment performance, verify process robustness, and ensure product quality and safety. Conduct thorough risk assessments, validation studies, and quality control tests to mitigate potential hazards and maintain product integrity throughout the manufacturing process.
Incorporating these comprehensive strategies into your bioreactor and fermentor operations can help optimize performance, ensure data integrity, and achieve consistent results in research and production processes.
Conclusion
Integrating bioreactors and fermentors into your scientific workflow represents a transformative step towards unlocking a multitude of benefits, ranging from enhanced efficiency and data quality to accelerated research and production timelines. By meticulously understanding your requirements, selecting the right equipment, optimizing integration processes, and implementing best practices, you can effectively harness the full potential of these powerful tools and drive innovation in your laboratory or production facility.
Partner with IT Tech for Seamless Integration
IT Tech stands as your trusted partner in the realm of bioreactors and fermentors, offering a comprehensive suite of high-quality equipment sourced from leading manufacturers. With our extensive expertise and dedication to customer satisfaction, we are committed to providing tailored solutions that meet your specific needs and propel your scientific endeavors to new heights. Partner with IT Tech today to embark on a journey of seamless integration and unparalleled innovation in your laboratory or production environment. Unlock the full potential of bioreactors and fermentors with IT Tech by your side.
Products You may Like
Check out other IT- Tech product that suit your taste
Subscribe to our newsletter
Stay updated with IT-Tech Insights
Related posts
Check out other IT- Tech Scientific Resources
Transforming Scientific Discovery with Bioreactors and Fermentors
Explore the transformative potential of bioreactors and fermentors in driving cutting-edge research across biotechnology, pharmaceuticals, and more. Discover the latest advancements shaping the future of scientific innovation.
.png)
Making the Right Investment: A Detailed Analysis of Bioreactors
Discover the definitive guide to making informed investments in bioreactors. Explore the technical nuances, economic considerations, and supplier partnerships crucial for maximizing returns in bioprocessing. From single-use to stainless steel, gain insights to navigate the complexities and optimize your lab or production facility with expert advice and tailored solutions.
.png)
Unlocking Full Potential: Advanced Uses for Fermentors
Unlock the full potential of fermentors with advanced sensors, precise environmental control, optimized hardware design, and intelligent automation strategies. Explore applications across industries, from biologics manufacturing to bioscience R&D, and discover how state-of-the-art fermentors can revolutionize your processes. Contact us to speak with bioprocessing experts and elevate your laboratory or production goals.













































































