Bioreactors and Fermentors: Transforming Research through Innovative Technology
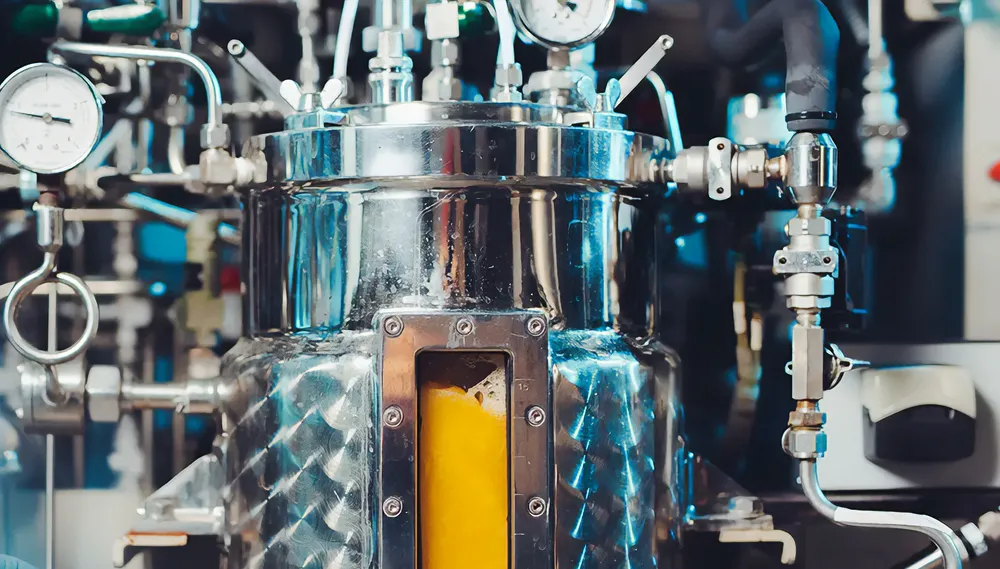
Bioreactors and fermentors are at the forefront of modern research and industrial processes, revolutionizing the way biological reactions are conducted. In this comprehensive guide, we delve into the intricacies of these innovative technologies, exploring their key features, working principles, types, applications, and the latest advancements driving transformative research across various industries.
What are Bioreactors and Fermentors?
Bioreactors and fermentors are sophisticated vessels meticulously engineered to create controlled environments conducive to biochemical reactions. While the terms are often used interchangeably, bioreactors encompass a broader scope, accommodating various biochemical reactions, including enzymatic reactions, cell cultures, microbial fermentations, and tissue engineering applications. Fermentors, on the other hand, are specialized bioreactors tailored specifically for microbial fermentation reactions, optimizing conditions for the rapid growth of microorganisms and the maximal production of desired metabolic products, such as enzymes and pharmaceuticals.
These vessels provide a sterile, monitored, and tightly controlled milieu where crucial parameters like temperature, pH, oxygen levels, and nutrient availability are meticulously regulated to cultivate cells, microorganisms, or tissues and produce desired compounds ranging from pharmaceuticals and food ingredients to biochemicals and biofuels.
Key Features and Working Principle
Components of Bioreactors and Fermentors:
- Vessel or Tank: The primary container where biological processes occur, typically constructed from materials like stainless steel or glass.
- Agitators: Mechanisms for mixing the reactor contents to ensure uniform distribution of nutrients, gases, and cells.
- Spargers: Devices used to disperse air or oxygen for aerobic cultures.
- Probes and Sensors: Instruments for real-time monitoring and control of critical parameters such as pH, temperature, pressure, and foam levels.
- Ports: Access points for adding nutrients, inoculum, reagents, and sampling.
- Controllers: Systems for maintaining optimal conditions by regulating temperature, pH, and other parameters.
- Pumps: Mechanisms for controlling the addition and removal of liquids, gases, and cells.
- Filters: Components for maintaining sterility by filtering incoming and outgoing air and nutrients.
Working Principle:
- Preparation of Culture Medium: The vessel is filled with a culture medium containing nutrients essential for cell growth and metabolism.
- Setting Optimal Parameters: Parameters like temperature, pH, and dissolved oxygen levels are set to optimal ranges conducive to the specific biological process.
- Inoculation: The culture medium is inoculated with cells or microorganisms, initiating the biological process.
- Monitoring and Control: Probes and sensors continuously monitor crucial parameters, which are adjusted as needed by controllers to maintain optimal conditions.
- Agitation and Aeration: Agitation mechanisms ensure thorough mixing of the contents, while spargers facilitate proper aeration, particularly in aerobic cultures.
- Sampling and Analysis: Periodic samples are withdrawn from the bioreactor for analysis, allowing researchers to monitor growth, metabolite production, and other relevant parameters.
- Harvesting and Purification: Once the desired level of growth or product formation is achieved, the contents are harvested and subjected to purification processes to obtain the final product.
By meticulously controlling these parameters, bioreactors and fermentors enable researchers and manufacturers to achieve maximum productivity and efficiency in their bioprocesses.
Major Types of Bioreactors
Bioreactors come in various designs, each tailored to suit specific biological systems and research objectives. Let's explore some of the major types:
1. Stirred-Tank Bioreactor

Source: Bioprocess International
Description:
Stirred-tank bioreactors, also known as stirred-tank reactors or simply STRs, represent the cornerstone of bioprocessing equipment due to their versatility and scalability. These bioreactors feature a cylindrical vessel equipped with impellers and spargers, facilitating efficient mixing and aeration throughout the culture medium. The impellers create turbulent flows, ensuring uniform distribution of nutrients, gases, and cells, while the spargers introduce air or gases for aerobic processes. Stirred-tank bioreactors are typically constructed from high-grade stainless steel or glass to withstand rigorous sterilization procedures.
Applications:
Stirred-tank bioreactors find widespread applications across various industries, including:
- Microbial Fermentation: Ideal for producing enzymes, antibiotics, and biochemicals using microbial cultures.
- Mammalian Cell Culture: Suitable for the production of recombinant proteins, monoclonal antibodies, and vaccines.
- Aerobic Waste Treatment: Used in wastewater treatment plants for aerobic microbial processes to degrade organic pollutants.
- Biopharmaceutical Manufacturing: Critical for large-scale production of biologics such as insulin and growth factors.
2. Airlift Bioreactor

Source: Pharmaguides
Description:
Airlift bioreactors offer an alternative to stirred-tank bioreactors, employing gas injection to induce circulation within the vessel. This design consists of a riser and a downcomer or draught tube, creating a looped flow pattern that promotes gentle mixing while ensuring efficient oxygen transfer. Unlike stirred-tank bioreactors, airlift bioreactors operate without mechanical agitation, reducing shear stress on delicate cells or microorganisms.
Applications:
Airlift bioreactors are particularly suited for processes requiring gentle mixing, such as:
- Cell Cultures: Suitable for cultivating animal or plant cells in suspension, minimizing cell damage and shear forces.
- Microbial Fermentations: Effective for the production of metabolites, enzymes, and biofuels using microbial cultures.
- Bioremediation: Utilized in environmental applications for biodegradation of contaminants in soil and water.
3. Packed Bed Bioreactor

Source: ResearchGate
Description:
Packed bed bioreactors feature a fixed-bed configuration packed with immobilized enzymes, cells, or catalysts, through which the medium or gas flows continuously. This design facilitates high-density cell growth and efficient mass transfer, allowing for enhanced productivity and process stability. Packed bed bioreactors can be constructed from various materials, including glass, stainless steel, or specialized polymers, depending on the specific application requirements.
Applications:
Packed bed bioreactors find diverse applications across industries, including:
- Wastewater Treatment: Utilized in biological treatment processes for the degradation of organic pollutants and removal of contaminants.
- Food Processing: Employed for the production of food additives, enzymes, and bioactive compounds through enzymatic conversions.
- Biocatalysis: Used in the synthesis of fine chemicals, pharmaceutical intermediates, and specialty products using immobilized enzymes or cells.
4. Hollow Fiber Bioreactor

Source: Researchgate
Description:
Hollow fiber bioreactors utilize bundles of hollow, porous fibers housed in a tubular casing to facilitate high-density cell culture in a compact system. Cells adhere to the exterior surface of the fibers, creating a large surface area for cell growth and interaction with the culture medium flowing through the fiber lumens. This design enables efficient nutrient exchange and waste removal, making hollow fiber bioreactors suitable for continuous perfusion cultures and long-term cell culture applications.
Applications:
Hollow fiber bioreactors are commonly employed in applications requiring high-density cell culture, including:
- Monoclonal Antibody Production: Used for large-scale production of therapeutic antibodies for pharmaceutical applications.
- Stem Cell Culture: Suitable for the expansion and differentiation of stem cells for regenerative medicine and tissue engineering.
- Vaccine Production: Utilized in the production of viral vaccines and viral vectors for gene therapy applications.
5. Photo Bioreactor

Source: Researchgate
Description:
Photo bioreactors harness light as an energy source for the cultivation of photosynthetic organisms, such as algae, cyanobacteria, and plant cells. These bioreactors are designed to optimize light exposure while providing a controlled environment for efficient biomass production. Photo bioreactors can vary in design, including flat-panel systems, tubular reactors, and enclosed photobioreactors, depending on the specific requirements of the photosynthetic organism and the desired application.
Applications:
Photo bioreactors are primarily used in applications involving photosynthetic organisms, including:
- Biofuel Production: Utilized for the production of biofuels, such as biodiesel and bioethanol, from microalgae or cyanobacteria.
- Nutraceutical Production: Employed for the cultivation of microalgae rich in omega-3 fatty acids, antioxidants, and other bioactive compounds for nutraceutical applications.
- Wastewater Treatment: Utilized for the removal of nutrients, heavy metals, and organic pollutants from wastewater through algal-based treatment systems.
Bioreactor Sensors
Bioreactor sensors play a pivotal role in monitoring and controlling critical parameters essential for optimizing bioprocesses. These sensors provide real-time data on parameters such as pH, dissolved oxygen, temperature, and nutrient concentrations, enabling precise control of bioreactor conditions to maximize productivity and process efficiency.
Common Types of Bioreactor Sensors:
- pH Sensors: Electrochemical and optical sensors for continuous pH monitoring, ensuring optimal pH conditions for cell growth and metabolite production.
- DO Sensors: Clark-type polarographic sensors for monitoring dissolved oxygen levels, essential for aerobic processes and cell viability.
- Optical Density Sensors: Devices for measuring turbidity to estimate cell density, providing insights into cell growth kinetics and biomass production.
- Temperature Sensors: Thermocouples and Resistance Temperature Detectors (RTDs) for temperature measurement, maintaining temperature stability for optimal bioprocess performance.
- Conductivity Sensors: Instruments for estimating nutrient concentrations, facilitating precise control of nutrient supplementation in the culture medium.
- Gas Analyzers: Equipment for analyzing outgoing gases, such as carbon dioxide, methane, and oxygen, providing insights into metabolic activity and gas exchange rates in the bioreactor.
Integration of advanced sensor technologies, including wireless sensors and multiparameter monitoring systems, allows for more efficient monitoring and control of bioprocess parameters, enhancing productivity, and process robustness. These sensors play a critical role in optimizing bioprocess conditions, minimizing variability, and ensuring consistent product quality in biopharmaceutical, food and beverage, and environmental applications.
Innovations in Bioreactor Technology
The field of bioreactor technology is undergoing a paradigm shift, driven by a relentless pursuit of efficiency, scalability, and adaptability. Let's delve into some of the most groundbreaking innovations transforming the landscape of bioprocessing:
Single-Use Bioreactors
Single-use bioreactors represent a revolutionary advancement in bioprocessing technology. These disposable systems eliminate the need for traditional stainless steel vessels, offering a sterile, ready-to-use solution for biomanufacturing. By eliminating cleaning and sterilization steps, single-use bioreactors significantly reduce cross-contamination risks and downtime between batches. Moreover, they enable greater flexibility in bioprocessing workflows, allowing for rapid process development and scale-up without the constraints of fixed infrastructure.
Microscale/Miniature Bioreactors
Miniaturized bioreactor platforms have emerged as indispensable tools for high-throughput experimentation and process optimization. These compact systems replicate the functionalities of traditional bioreactors on a smaller scale, allowing researchers to simultaneously conduct numerous experiments in parallel. Microscale bioreactors enable rapid screening of culture conditions, media formulations, and bioprocess parameters, accelerating the pace of bioprocess development. Furthermore, they facilitate seamless scale-up studies, providing valuable insights into process scalability and performance across different production scales.
Bioprinted Tissue Constructs
The advent of 3D bioprinting technologies has ushered in a new era of tissue engineering and regenerative medicine. By precisely depositing bioinks composed of living cells and biomaterials, bioprinters can fabricate intricate tissue constructs with tailored architectures and functionalities. These bioprinted tissues hold immense potential for applications in drug testing, disease modeling, and tissue regeneration. Researchers can create physiologically relevant tissue models to study disease mechanisms, screen drug candidates, and personalize medical treatments. Bioprinted tissues also offer promise for regenerating damaged or diseased tissues, paving the way for advanced therapies in regenerative medicine.
Automation
Automation has become increasingly integral to bioprocess operations, streamlining workflows and enhancing reproducibility. Automated bioreactor systems are equipped with sophisticated monitoring, control, and data logging capabilities, enabling precise regulation of bioprocess parameters. Automated workflows minimize human intervention, reducing the risk of errors and variability in experimental outcomes. Furthermore, automated data acquisition and analysis facilitate real-time process monitoring and decision-making, optimizing process performance and resource utilization. From seed inoculation to harvest, automation accelerates bioprocesses, improving efficiency and productivity across the biomanufacturing continuum.
Bioreactor Data Analytics
The integration of advanced instrumentation and bioinformatics tools has revolutionized the analysis of bioreactor data. By harnessing big data analytics and machine learning algorithms, researchers can extract valuable insights from complex bioprocess datasets. Bioreactor data analytics enable real-time monitoring of key performance indicators, trend analysis, and predictive modeling of process outcomes. These insights empower researchers to optimize bioprocess parameters, identify potential bottlenecks, and drive continuous improvement. By leveraging data-driven decision-making, biopharmaceutical companies can enhance process robustness, accelerate time-to-market, and ensure the quality and consistency of biologic products.
Continuous Bioprocessing
Continuous bioprocessing has emerged as a game-changing approach to biomanufacturing, offering numerous advantages over traditional batch processes. By enabling uninterrupted operation and in-line monitoring, continuous bioprocessing delivers consistent product quality, enhanced productivity, and reduced manufacturing footprint. Continuous bioprocesses eliminate the need for large-scale batch reactors and prolonged downtimes associated with cleaning and sterilization cycles. Instead, they allow for continuous product harvest, resulting in higher overall yields and shorter production cycles. Moreover, continuous bioprocessing enables real-time process monitoring and control, facilitating rapid adjustments to optimize process performance and resource utilization.
These innovations represent the cutting edge of bioreactor technology, driving transformative changes across biomedical and bioprocess engineering disciplines. By harnessing the power of single-use systems, miniaturized platforms, bioprinted tissues, automation, data analytics, and continuous processing, researchers and manufacturers are poised to achieve unprecedented levels of efficiency, productivity, and innovation in bioprocessing.
Applications of Bioreactors and Fermentors
The versatility of bioreactors and fermentors extends across a myriad of industries and applications, revolutionizing bioproduction and catalyzing innovation. Let's explore some of the key applications:
Biopharmaceutical Production
Bioreactors are the workhorses of biopharmaceutical manufacturing, enabling the production of antibodies, vaccines, recombinant proteins, and other biologics. Mammalian and bacterial cell cultures cultivated in bioreactors serve as biofactories for the large-scale production of therapeutic proteins and vaccines. From insulin and growth factors to monoclonal antibodies and viral vectors, bioreactors play a pivotal role in meeting the growing demand for biopharmaceuticals worldwide.
Tissue Engineering
Bioreactors provide the ideal environment for the cultivation of functional 3D tissues for applications in regenerative medicine and tissue engineering. Researchers can seed cells onto scaffold materials within bioreactor systems, providing mechanical support and guiding tissue development. From cartilage and skin to bone and vascular tissues, bioreactor-grown tissues hold promise for repairing and regenerating damaged or diseased organs and tissues.
Stem Cell Expansion
Bioreactors facilitate the large-scale expansion of stem cells for therapeutic applications while preserving their differentiation potential. Controlled culture conditions within bioreactors promote cell proliferation and maintain stem cell potency, ensuring the generation of a sufficient cell population for cell-based therapies. Stem cell bioprocessing holds significant potential for treating a wide range of diseases and injuries, from neurodegenerative disorders to cardiovascular diseases and spinal cord injuries.
Microbial Metabolism
Microbial fermentation in bioreactors serves as a cost-effective and sustainable approach to the production of enzymes, organic acids, alcohols, antibiotics, and other biochemicals. Microorganisms cultivated in bioreactors metabolize substrates to generate valuable products, offering alternative pathways to traditional chemical synthesis. From industrial enzymes and biofuels to pharmaceutical intermediates and specialty chemicals, microbial fermentation continues to drive innovation across various sectors.
Wastewater Treatment
Bioreactors play a crucial role in biological wastewater treatment processes, facilitating the degradation of organic pollutants and the removal of contaminants from sewage and industrial wastewater. Aerobic and anaerobic bioreactors harbor microbial communities capable of metabolizing organic compounds, transforming pollutants into harmless byproducts. Bioremediation technologies based on bioreactors offer sustainable solutions for environmental remediation, preserving water quality and ecosystem health.
Food Production
Fermentation-based production in bioreactors enables the synthesis of food ingredients such as probiotics, vitamins, organic acids, and enzymes. Microorganisms cultivated in bioreactors ferment raw materials to produce value-added products with enhanced nutritional profiles and functional properties. From dairy products and fermented beverages to plant-based proteins and nutraceuticals, bioreactor-derived ingredients play a vital role in the food and beverage industry, meeting consumer demand for healthy and sustainable food options.
Biofuel Production
Bioreactors serve as the cornerstone of biofuel production processes, enabling the cultivation of microalgae for the production of oils, biodiesel, bioethanol, and biogas. Microalgae cultivated in bioreactors harness solar energy through photosynthesis to convert carbon dioxide into biomass rich in lipids and carbohydrates. These biomass feedstocks can be processed into renewable fuels with lower carbon footprints compared to fossil fuels, offering sustainable alternatives for transportation and energy production.
The applications of bioreactors and fermentors continue to evolve and expand, driven by advancements in technology and emerging market demands. As researchers and manufacturers unlock new possibilities and address emerging challenges, bioreactors will remain at the forefront of innovation, shaping the future of bioproduction and sustainable development.
Conclusion
Bioreactors and fermentors represent the pinnacle of modern bioprocessing technology, empowering researchers and manufacturers to conduct sophisticated biochemical reactions with precision and efficiency. From biopharmaceutical production to tissue engineering and environmental remediation, these innovative systems play a vital role in driving scientific advancements and industrial innovation.
At IT Tech, we understand the critical importance of bioreactors in research and industrial processes. With our comprehensive range of bioreactor solutions, including stirred-tank, airlift, packed bed, hollow fiber, and photo bioreactors, we are committed to providing cutting-edge technologies tailored to meet the diverse needs of our customers. Whether you're conducting fundamental research in the laboratory or scaling up bioproduction in a manufacturing facility, our expertise and support services ensure optimal performance and productivity at every stage of your bioprocess journey.
Ready to revolutionize your research and projects with state-of-the-art bioreactor solutions? Contact us today to learn more about how IT Tech can accelerate your scientific endeavors and drive innovation in your industry.
Submit an enquiry to learn how our bioreactors and fermentors can accelerate your research and projects.
Products You may Like
Check out other IT- Tech product that suit your taste
Subscribe to our newsletter
Stay updated with IT-Tech Insights
Related posts
Check out other IT- Tech Scientific Resources
.png)
Integrating Bioreactors & Fermentors for Lab Efficiency
Discover expert tips and best practices for seamlessly integrating bioreactors and fermentors into your laboratory workflow. From selecting the right equipment to optimizing growth conditions, elevate your scientific endeavors with our comprehensive guide.
.png)
Making the Right Investment: A Detailed Analysis of Bioreactors
Discover the definitive guide to making informed investments in bioreactors. Explore the technical nuances, economic considerations, and supplier partnerships crucial for maximizing returns in bioprocessing. From single-use to stainless steel, gain insights to navigate the complexities and optimize your lab or production facility with expert advice and tailored solutions.
.png)
Unlocking Full Potential: Advanced Uses for Fermentors
Unlock the full potential of fermentors with advanced sensors, precise environmental control, optimized hardware design, and intelligent automation strategies. Explore applications across industries, from biologics manufacturing to bioscience R&D, and discover how state-of-the-art fermentors can revolutionize your processes. Contact us to speak with bioprocessing experts and elevate your laboratory or production goals.










































































