Top Bioreactors & Fermentors Tools for Modern Labs: Powering Discovery & Innovation

The world of bioreactors and fermentors is undergoing a revolution. From drug discovery to biofuels, these advanced tools are at the heart of groundbreaking scientific achievements. As a lab technician, project manager, procurement manager, or C-suite leader in the scientific industry, choosing the right bioreactor or fermentor is crucial for your research success.

This comprehensive guide dives into the top bioreactors and fermentors tools for modern labs, equipping you with the knowledge to make informed decisions.
Why Bioreactors & Fermentors are Essential Tools
Bioreactors and fermentors serve as vital tools in various scientific and industrial applications, providing controlled environments crucial for cultivating cells and microorganisms. Their versatility makes them indispensable across multiple fields:
- Drug Discovery and Development: Bioreactors play a central role in pharmaceutical research by facilitating the production of recombinant proteins, antibodies, and other biopharmaceuticals. These essential components are critical for developing new drugs, vaccines, and therapeutic agents aimed at treating various diseases and medical conditions.
- Biofuels and Renewable Energy: In the quest for sustainable energy sources, bioreactors are instrumental in creating biofuels such as ethanol and biodiesel from renewable sources like biomass, algae, and agricultural waste. Through fermentation processes, microorganisms can efficiently convert organic matter into biofuels, offering a cleaner and greener alternative to fossil fuels.
- Food and Beverage Production: Bioreactors are widely used in the food and beverage industry for fermenting various products, including beer, wine, yogurt, and other fermented foods. Controlled fermentation processes enhance flavor development, improve shelf life, and create desirable textures, making bioreactors indispensable in food production.
- Environmental Applications: Bioreactors play a crucial role in environmental remediation efforts by facilitating the biodegradation of pollutants and wastewater treatment. Microorganisms cultivated within bioreactors can metabolize contaminants, transforming them into less harmful substances and contributing to the restoration of polluted ecosystems.
- Biomaterials Development: In regenerative medicine and tissue engineering, bioreactors are essential for engineering tissues and organs for transplantation. By providing the necessary environmental cues and stimuli, bioreactors enable the growth, differentiation, and organization of cells into functional tissues, offering promising solutions for treating injuries and diseases.
- Basic Research: Bioreactors are invaluable tools for fundamental research aimed at understanding cellular processes and metabolic pathways. By providing a controlled environment for culturing cells and microorganisms, bioreactors enable researchers to study cell behavior, gene expression, and biochemical reactions, advancing our knowledge of biology and biochemistry.
Bioreactors and fermentors come in a wide range of sizes, configurations, and functionalities, providing versatility to meet diverse research and production requirements. Selecting the appropriate tool involves careful consideration of various factors:
- Scale: Bioreactors and fermentors are available in benchtop, pilot-scale, and production-scale capacities. Benchtop systems are suitable for small-scale experiments and research, offering flexibility and ease of use. Pilot-scale bioreactors are designed for intermediate-scale production or process optimization, providing a bridge between laboratory-scale and full-scale manufacturing. Production-scale bioreactors are large-scale systems tailored for commercial production, accommodating higher volumes and throughput.
- Type of Culture: Different bioreactor designs are optimized for specific types of cultures, including microbial, mammalian, plant, and insect cells. Microbial bioreactors are commonly used for bacterial and yeast cultures, while mammalian cell cultures require specialized systems to meet stringent requirements for cell growth and product yield. Plant bioreactors cater to the cultivation of plant cells or tissues for pharmaceutical or agricultural applications.
- Desired Outputs: The choice of bioreactor depends on the desired outputs, which may include biomass, proteins, metabolites, enzymes, or other bioproducts. Each bioreactor type may offer advantages in terms of product yield, purity, and scalability, depending on the specific requirements of the application.
- Process Requirements: Bioreactors must meet specific process requirements to ensure optimal performance and product quality. Key considerations include sterility, temperature control, oxygenation, pH control, agitation, and nutrient supply. Bioreactors equipped with advanced control systems and sensors allow precise regulation of these parameters, facilitating optimal growth conditions for cells or microorganisms.
- Budget and Available Resources: Budget constraints and available resources play a significant role in selecting the right bioreactor. While advanced bioreactor systems may offer enhanced features and performance, they often come with higher costs. It's essential to balance the upfront investment with long-term benefits and consider factors such as maintenance requirements, consumables, and operational expenses.
Top Bioreactors & Fermentors Tools for Your Lab
- Stirred-tank bioreactors (STRs)
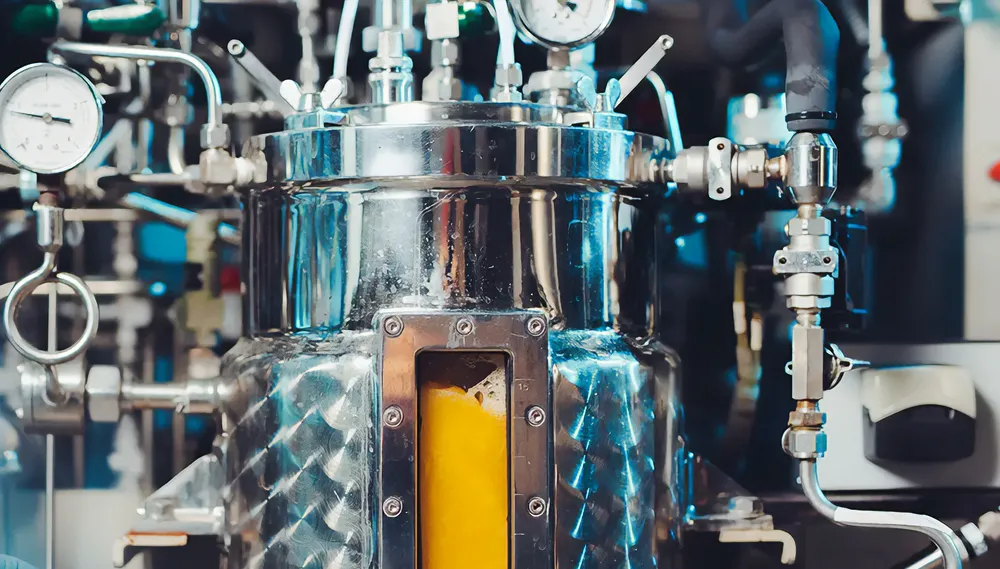
Stirred-tank bioreactors (STRs) represent a cornerstone in bioprocessing due to their versatility and widespread use across various industries. These workhorses are particularly favored for microbial and cell cultures, owing to their ability to provide optimal conditions for cell growth and product formation.
At the heart of STRs lies their agitators, which play a crucial role in mixing the culture broth and ensuring homogeneity. This agitation mechanism facilitates the dispersion of nutrients, gases, and other essential components throughout the culture, promoting uniform growth and metabolite production. Additionally, agitation helps prevent the formation of gradients in parameters such as temperature, pH, and dissolved oxygen, which could compromise cell viability and productivity.
The design of STRs allows for flexibility in operation, enabling researchers and bioprocess engineers to tailor operating conditions to specific requirements. Parameters such as agitation speed, aeration rate, temperature, and pH can be precisely controlled and optimized to maximize cell growth and product yield.

Examples of prominent stirred-tank bioreactors include the Applikon Biotron, Sartorius BIOSTAT STR, and Thermo Scientific BioFlo. These systems are renowned for their robust construction, reliable performance, and user-friendly interfaces, making them ideal choices for both research and industrial applications.
In addition to their primary function of supporting cell growth and bioproduction, STRs often integrate advanced features for process monitoring and control. Real-time monitoring of key parameters allows for timely adjustments to optimize performance and ensure consistent product quality.
Overall, stirred-tank bioreactors continue to be indispensable tools in bioprocessing, offering unparalleled versatility, reliability, and scalability. Their widespread adoption underscores their critical role in driving advancements in biotechnology, pharmaceuticals, and other related fields.
- Airlift bioreactors
Airlift bioreactors represent a distinct category of bioreactor designs renowned for their utilization of air bubbles to facilitate mixing within the culture medium. Unlike traditional stirred-tank bioreactors that rely on mechanical agitation, airlift bioreactors utilize the buoyancy of air bubbles to induce circulation, creating a gentle and uniform mixing environment. This unique mixing mechanism makes airlift bioreactors particularly well-suited for applications involving shear-sensitive cells, where excessive agitation can lead to cell damage or loss of viability.
One of the key advantages of airlift bioreactors lies in their ability to provide efficient oxygen transfer to the culture medium. The upward flow of air bubbles not only promotes mixing but also facilitates the exchange of gases, ensuring adequate oxygenation of the cells or microorganisms. This is crucial for aerobic processes such as cell growth and protein expression, where oxygen availability directly impacts cellular metabolism and productivity.
Airlift bioreactors are favored in various bioprocess applications, including the cultivation of mammalian cells, plant cells, and certain types of microbial cultures. Their gentle mixing action minimizes shear forces on delicate cells, preserving their integrity and enhancing overall cell growth and productivity. Additionally, airlift bioreactors offer advantages in scalability, as the basic operating principles remain consistent across different reactor sizes, allowing for seamless transition from laboratory-scale experiments to industrial-scale production.

Two notable examples of airlift bioreactors include the INFORS HT High Throughput Screening Reactors and the Sartorius BIOSTAT DCU. The INFORS HT reactors are specifically designed for high-throughput screening applications, enabling rapid and parallel cultivation of multiple cell lines or microbial strains. These reactors offer precise control over operating parameters and are well-suited for applications requiring stringent process optimization.
Similarly, the Sartorius BIOSTAT DCU airlift bioreactor platform is renowned for its versatility and scalability. Equipped with advanced control systems and monitoring capabilities, the BIOSTAT DCU series enables precise regulation of process parameters such as temperature, pH, and dissolved oxygen levels. This ensures optimal growth conditions for a wide range of cell types and facilitates the production of high-quality bioproducts.
In summary, airlift bioreactors offer a compelling combination of gentle mixing, efficient oxygen transfer, and scalability, making them indispensable tools in bioprocess engineering. With their ability to accommodate shear-sensitive cells and provide optimal growth conditions, airlift bioreactors contribute significantly to the advancement of biopharmaceutical manufacturing, cell therapy development, and other biotechnology applications.
- Wave bioreactors
Wave bioreactors are a specialized type of bioreactor that operate on the principle of gentle rocking motion, which distinguishes them from traditional stirred-tank bioreactors. This unique rocking motion creates a wave-like motion within the culture vessel, facilitating efficient mixing and aeration while minimizing shear stress on cells and microcarriers.
One of the key advantages of wave bioreactors is their ability to provide a controlled environment for the cultivation of fragile cells and microorganisms. The gentle rocking motion helps to maintain cell viability and functionality, making wave bioreactors particularly well-suited for applications that involve sensitive cell types or delicate structures, such as stem cells, primary cells, and tissue engineering constructs.
Wave bioreactors offer several benefits over conventional bioreactor systems. The absence of mechanical agitation reduces the risk of shear-induced cell damage, resulting in higher cell yields and improved product quality. Additionally, the wave-like motion promotes uniform mixing and oxygen transfer throughout the culture, ensuring consistent growth conditions and enhanced process reproducibility.

Two notable examples of wave bioreactor systems are the WAVE Bioreactor System and the GE Healthcare WAVE Bioreactor 2000. These systems are designed to accommodate various vessel sizes and configurations, allowing flexibility in scale-up and process optimization. The WAVE Bioreactor System, developed by GE Healthcare, features disposable cell culture bags that simplify operation and minimize contamination risks, making it an attractive option for research and production environments.
In summary, wave bioreactors offer a gentle and efficient solution for the cultivation of fragile cells and microcarriers. Their unique rocking motion minimizes shear stress while promoting uniform mixing and oxygen transfer, making them an invaluable tool for a wide range of applications in biopharmaceuticals, regenerative medicine, and bioprocessing. With advancements in technology and design, wave bioreactors continue to play a vital role in driving innovation and progress in the field of biotechnology.
- Membrane bioreactors (MBRs)
Membrane bioreactors (MBRs) represent a cutting-edge technology that merges biodegradation processes with membrane filtration for highly efficient wastewater treatment and bioproduct recovery. This innovative approach addresses the growing global demand for sustainable and environmentally friendly wastewater treatment solutions. MBRs offer several advantages over conventional treatment methods, making them increasingly popular in various industrial, municipal, and residential applications.
Principle of Operation: MBRs combine biological treatment processes, such as activated sludge or biofilm reactors, with membrane filtration. Wastewater is first subjected to biological degradation, where microorganisms break down organic pollutants into simpler compounds. The treated effluent then passes through ultrafiltration or microfiltration membranes, which effectively remove suspended solids, bacteria, and pathogens, producing high-quality treated water.
Efficiency and Performance: MBRs provide superior treatment performance compared to conventional wastewater treatment processes. The membrane barrier ensures a physical barrier to contaminants, resulting in effluent of consistently high quality with low turbidity and suspended solids levels. MBRs are capable of removing a wide range of pollutants, including organic matter, nutrients, pathogens, and emerging contaminants, meeting stringent discharge standards and environmental regulations.
Bioproduct Recovery: In addition to wastewater treatment, MBRs offer the potential for bioproduct recovery. The concentrated biomass retained by the membrane can be harvested and processed to extract valuable resources, such as bioenergy (biogas), biofertilizers, biopolymers, and other bioproducts. This integrated approach enhances resource recovery and valorization, contributing to the circular economy and sustainable resource management.

Examples of MBR Systems: Several manufacturers offer MBR systems tailored to different applications and treatment capacities. Prominent examples include the GE ZeeWeed MBR and the Kubota MBR Membrane Bioreactor System. These systems feature robust membrane modules, advanced control systems, and modular designs to optimize performance, ease of operation, and maintenance.
Applications: MBRs find applications in various sectors, including municipal wastewater treatment, industrial wastewater treatment (e.g., food and beverage, pharmaceuticals, chemical manufacturing), decentralized wastewater treatment in remote or rural areas, and water reuse/recycling schemes. Their versatility and scalability make them suitable for both small-scale and large-scale installations, catering to diverse treatment needs.
In summary, membrane bioreactors represent a sophisticated and efficient solution for wastewater treatment and bioproduct recovery. With their advanced technology, MBRs offer unparalleled treatment performance, resource recovery potential, and environmental sustainability, contributing to the advancement of circular economy principles and the preservation of water resources.
- Single-use bioreactors
Single-use bioreactors represent a significant advancement in bioprocessing technology, offering numerous benefits over traditional stainless steel systems. These innovative disposable bioreactors eliminate the need for time-consuming and resource-intensive cleaning and sterilization processes, streamlining operations and saving valuable time and labor. By eliminating the risk of cross-contamination between batches, single-use bioreactors also reduce the potential for product loss and ensure higher product quality and consistency.

Two prominent examples of single-use bioreactors are the Thermo Scientific HyPerforma Single-Use Bioreactors and the Sartorius ambr® 250 Single-Use Bioreactor. The Thermo Scientific HyPerforma system is renowned for its scalability, allowing seamless transition from benchtop to production-scale processes. Its robust design and efficient mixing capabilities make it suitable for a wide range of cell culture applications. On the other hand, the Sartorius ambr® 250 Single-Use Bioreactor offers high-throughput capabilities, enabling parallel processing of multiple cultures in miniature bioreactor vessels. This system is particularly advantageous for process development and optimization, facilitating rapid iteration and decision-making.
In addition to their operational advantages, single-use bioreactors offer enhanced flexibility, allowing researchers to adapt quickly to changing experimental requirements. They are particularly well-suited for applications where product changeover is frequent or where facility space is limited. Moreover, single-use systems significantly reduce the risk of contamination, thereby enhancing product safety and regulatory compliance.
As the biopharmaceutical industry continues to evolve, single-use bioreactors are poised to play an increasingly critical role in bioprocessing workflows. Their ability to streamline operations, improve product quality, and reduce costs makes them a compelling choice for modern biomanufacturing facilities. With ongoing advancements in materials science and engineering, single-use bioreactors are likely to become even more versatile and efficient in the years to come, driving further innovation in the field of bioprocessing.
Advanced technologies
Advanced technologies in the realm of bioreactors offer innovative solutions to enhance productivity and flexibility in laboratory settings:
- Perfusion Bioreactors:
- Perfusion bioreactors represent a significant advancement in cell culture technology by enabling continuous culture systems. Unlike traditional batch or fed-batch processes, perfusion bioreactors provide a constant flow of fresh media while simultaneously removing spent media and waste products. This continuous exchange sustains high-density cell growth over extended periods, resulting in increased product yields. Perfusion systems are particularly advantageous for applications requiring tightly controlled environmental conditions and prolonged cultivation times, such as the production of recombinant proteins, antibodies, and viral vectors for gene therapy. By mimicking the dynamic conditions found in vivo, perfusion bioreactors offer enhanced physiological relevance and scalability, making them indispensable tools in biopharmaceutical manufacturing and regenerative medicine research.
- Photobioreactors:
- Photobioreactors harness the power of light to cultivate photosynthetic microorganisms, such as algae and cyanobacteria, for various biotechnological applications. By optimizing light exposure, temperature, and nutrient availability, photobioreactors facilitate the efficient production of biofuels, including biodiesel and bioethanol, as well as high-value compounds like pigments, omega-3 fatty acids, and antioxidants. Additionally, photobioreactors play a vital role in environmental remediation by sequestering carbon dioxide from industrial flue gases and wastewater streams. Their ability to convert CO2 into biomass through photosynthesis mitigates greenhouse gas emissions while generating valuable biomass for renewable energy and sustainable feedstock production. Moreover, photobioreactors offer scalability and modularity, allowing researchers to tailor cultivation conditions to specific strains and optimize productivity for diverse applications in biorefineries, agriculture, and pharmaceuticals.
- Microfluidic Bioreactors:
- Microfluidic bioreactors leverage microfabrication techniques to create miniature and precisely controlled environments for cell culture and biochemical analysis. By confining cells within microscale channels and chambers, microfluidic devices offer unique advantages, including rapid mass transport, high surface-to-volume ratios, and precise spatiotemporal control over biochemical gradients. These features enable high-throughput screening of cellular responses to various stimuli, such as drugs, growth factors, and environmental cues, with minimal sample consumption and waste generation. Microfluidic bioreactors find applications in drug discovery, personalized medicine, tissue engineering, and fundamental research, where they enable real-time monitoring of cellular dynamics, single-cell analysis, and organ-on-a-chip models. Furthermore, their compatibility with advanced imaging and analytical techniques facilitates integration with computational models for predictive biology and systems-level understanding of complex biological processes.
Beyond the Hardware: Software & Monitoring
In addition to the hardware components, the integration of advanced software and monitoring systems has become a cornerstone of modern bioreactors and fermentors. These sophisticated software platforms play a pivotal role in controlling the intricate processes within these systems, facilitating data acquisition, analysis, and real-time monitoring.
The utilization of software in bioreactor and fermentor operations offers several key advantages. Firstly, it enables precise control over crucial parameters such as temperature, pH levels, dissolved oxygen, agitation speed, and nutrient supply. This precise control is essential for optimizing cell growth, product formation, and overall process efficiency.
Moreover, advanced software solutions provide researchers with the capability to collect and analyze vast amounts of data generated during bioprocessing. By harnessing data analytics tools, scientists can gain valuable insights into process performance, identify potential bottlenecks, and refine process parameters to enhance productivity and product quality.
Real-time monitoring capabilities offered by these software platforms are instrumental in ensuring process consistency and reproducibility. Continuous monitoring of critical parameters allows for prompt detection of deviations from desired conditions, enabling timely intervention and adjustment to maintain optimal process conditions. This proactive approach minimizes the risk of process failures and maximizes yield and product consistency.
Furthermore, software integration facilitates automation of various tasks within the bioprocess, streamlining workflow and reducing the need for manual intervention. This automation not only improves operational efficiency but also minimizes the potential for human error, contributing to overall process robustness and reliability.
In summary, the integration of advanced software and monitoring systems into bioreactors and fermentors represents a significant advancement in bioprocessing technology. These software solutions empower researchers with precise control, data-driven insights, and real-time monitoring capabilities, ultimately driving improvements in process efficiency, productivity, and product quality. As such, they have become indispensable tools in the modern laboratory, enabling researchers to push the boundaries of scientific discovery and innovation.
Choosing the Right Bioreactor/Fermentor: Expert Guidance is Key
Choosing the right bioreactor or fermentor is a critical decision that can significantly impact the success of your research or production processes. Given the complexity of the options available and the diverse needs of different applications, it's essential to seek expert guidance to ensure you make the most informed choice. This is where collaborating with a trusted supplier like IT Tech can make all the difference.
IT Tech stands out as a reliable partner in the bioreactor and fermentor industry, offering a range of benefits to support your specific requirements:
- Extensive Product Portfolio: With access to leading brands and a diverse range of technologies, IT Tech provides a comprehensive product portfolio to cater to your unique needs. Whether you're working in drug discovery, biofuel production, food and beverage fermentation, environmental applications, or any other field requiring bioreactors or fermentors, IT Tech has the right solution for you.
- Application Expertise: The team at IT Tech comprises experienced scientists who possess deep knowledge and understanding of various applications. They appreciate the challenges you face in your work and can offer valuable insights and recommendations to address your specific requirements. Whether you're dealing with microbial cultures, mammalian cells, plant cells, or any other type of culture, IT Tech's experts can guide you towards the most suitable solution.
- Technical Support: Acquiring a bioreactor or fermentor is just the beginning of your journey. To ensure smooth operation and optimal performance, comprehensive technical support is essential. IT Tech provides installation services, thorough training for your team, and ongoing troubleshooting assistance to address any issues that may arise. With IT Tech by your side, you can have confidence in the reliability and efficiency of your equipment.
- Financing Options: Investing in bioreactors or fermentors can be a significant financial commitment. Recognizing this, IT Tech offers flexible financing solutions to make acquiring the equipment you need more manageable. Whether you prefer outright purchase, lease options, or other financing arrangements, IT Tech works with you to find a solution that aligns with your budget and financial preferences.
Products You may Like
Check out other IT- Tech product that suit your taste
Subscribe to our newsletter
Stay updated with IT-Tech Insights
Related posts
Check out other IT- Tech Scientific Resources
.png)
Integrating Bioreactors & Fermentors for Lab Efficiency
Discover expert tips and best practices for seamlessly integrating bioreactors and fermentors into your laboratory workflow. From selecting the right equipment to optimizing growth conditions, elevate your scientific endeavors with our comprehensive guide.
Transforming Scientific Discovery with Bioreactors and Fermentors
Explore the transformative potential of bioreactors and fermentors in driving cutting-edge research across biotechnology, pharmaceuticals, and more. Discover the latest advancements shaping the future of scientific innovation.
.png)
Making the Right Investment: A Detailed Analysis of Bioreactors
Discover the definitive guide to making informed investments in bioreactors. Explore the technical nuances, economic considerations, and supplier partnerships crucial for maximizing returns in bioprocessing. From single-use to stainless steel, gain insights to navigate the complexities and optimize your lab or production facility with expert advice and tailored solutions.






















































.png)















































