Bioreactors vs. Fermentors: Which One Should You Pick? Navigating the Complexities of Bioreactors and Fermentors

Introduction: Unveiling the World of Bioreactors and Fermentors

Bioreactors and fermentors play pivotal roles in various industries such as pharmaceuticals, food processing, agriculture, environmental protection, and biofuel production. These advanced pieces of equipment enable researchers and manufacturers to harness the power of biological systems to produce valuable products like vaccines, enzymes, antibiotics, biofuels, and specialty chemicals. However, understanding which one is best suited for your specific application can be challenging due to their overlapping functionalities and terminologies. This article will provide an overview of these two essential tools, delve into their critical differences, guide you through the decision-making process, and introduce you to our diverse portfolio of solutions.
1.1 What are Bioreactors?

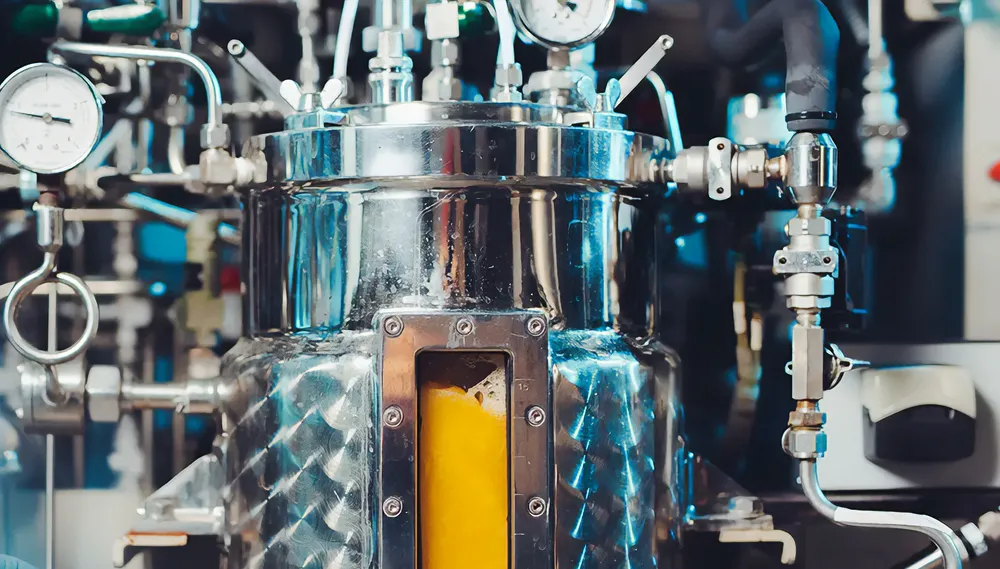
Bioreactors refer to vessels designed for cultivating cells, including plant, animal, insect, fungal, algae, and bacterial cells, under controlled conditions. By providing optimal temperature, pH, dissolved oxygen levels, nutrients, and other growth factors, bioreactors facilitate large-scale expansion and maintenance of living organisms while ensuring consistent output quality and yield. They serve as indispensable workhorses across numerous fields, from regenerative medicine to industrial manufacturing.
1.2 What are Fermentors?
Fermentors represent a subset of bioreactors specifically engineered for conducting fermentation processes – metabolic transformations carried out by microorganisms or enzymatic reactions resulting in desired chemical conversions. Historically, fermentation has been associated with traditional practices such as bread making, winemaking, and beer brewing; however, it now extends far beyond artisanal domains to support cutting-edge research and commercial-scale productions.
1.3 A Historical Perspective: From Ancient Practices to Modern Marvels

While human civilization's rudimentary experiments with fermentation date back thousands of years, contemporary bioreactors and fermentors have evolved significantly over time, incorporating sophisticated technologies that allow precise control over operational parameters, real-time monitoring capabilities, and seamless scalability. As science advances, so too does the potential for bioreactors and fermentors to revolutionize how we approach healthcare, sustainability, and technological innovation.
Delving Deeper: Key Differences Between Bioreactors and Fermentors
To better understand when to employ either a bioreactor or fermentor, let us examine their primary distinctions:
2.1 Operational Principles and Processes
Although both bioreactors and fermentors operate based on similar principles – manipulating physical and chemical variables within a closed system – they differ in terms of intended outcomes. Bioreactors focus primarily on expanding viable populations of cells, tissues, or organoids while maintaining ideal environments for their survival and proliferation. Meanwhile, fermentors prioritize maximizing the conversion of substrates into targeted compounds via enzymatic catalysis or microbial metabolism.
2.2 Target Organisms and Products
The choice between a bioreactor and fermentor often depends on whether the objective involves working with eukaryotic (animal, plant, or fungal) cells or prokaryotic (bacteria) species. Eukaryotic cells generally require more delicate handling than bacteria, necessitating specialized bioreactors equipped with features tailored to minimize shear stress and ensure uniform distribution of nutrients. In contrast, fermentors typically accommodate robust microbes capable of producing desired metabolites in substantial quantities without stringent concerns regarding mechanical damage during agitation.
2.3 Design and Features
Design aspects also distinguish bioreactors from fermentors. For instance, bioreactors may feature impellers, baffles, spargers, or membranes to promote adequate mixing and gas exchange, whereas fermentors might include additional components such as cooling jackets or coils to maintain lower temperatures required for certain metabolic pathways. Moreover, fermentors often contain antifoam agents to prevent excessive foam formation caused by vigorous aeration and agitation necessary for rapid growth and compound synthesis.
2.4 Applications and Industries
Finally, industry trends reveal distinct use cases for bioreactors versus fermentors. While both find widespread adoption across various sectors, some notable examples include:
- Pharmaceutical companies leveraging mammalian cell culture bioreactors to manufacture monoclonal antibodies, gene therapies, and viral vectors.
- Breweries utilizing oversized fermentors to churn out vast volumes of beer efficiently.
- Agricultural firms deploying plant tissue culture bioreactors to propagate elite crop varieties or generate secondary metabolites used in fertilizers and pesticides.
- Environmental agencies relying on wastewater treatment plants equipped with microbial bioreactors to break down pollutants and recycle resources.
Now that we have explored crucial dissimilarities between bioreactors and fermentors let us discuss essential factors guiding your selection process.
Making the Right Choice: Factors to Consider When Selecting Your Bioreactor or Fermentor
When deciding which type of bioreactor or fermentor would best suit your needs, consider the following criteria:
3.1 Scale and Production Volume
Determine whether you need a small-scale device for R&D purposes or a large-scale unit suitable for industrial-level output. Our extensive range includes benchtop models perfect for early-stage experimentation up to pilot-plant and production-scale systems capable of meeting demanding commercial requirements.
3.2 Desired Output: Cells, Metabolites, or Both?
Clarify if your goal entails growing healthy cells, generating specific metabolites, or achieving both objectives simultaneously. Different configurations cater to varying priorities, allowing optimized performance depending on your unique situation.
3.3 Organism or Cell Type Compatibility
Ensure compatibility between the chosen bioreactor/fermentor design and target organism(s). Some devices excel at supporting fragile mammalian cells, while others thrive when hosting hardy bacteria or yeasts.
3.4 Operating Parameters and Control Needs
Assess the level of precision needed to regulate critical operational parameters like temperature, pH, dissolved oxygen tension, and fluid dynamics. Advanced controllers integrated into our offerings empower users to maintain tight control ranges and fine-tune settings effortlessly.
3.5 Regulatory Requirements and Budgetary Constraints
Lastly, weigh regulatory compliance obligations against budget limitations before finalizing your selection. We strive to deliver cost-effective yet reliable solutions compliant with pertinent standards governing safety, efficacy, and documentation.
Exploring the Diverse Landscape: Types of Bioreactors and Fermentors

Our catalog boasts several categories catering to diverse applications:
4.1 Stirred-Tank Bioreactors: The Workhorses of the Industry
Stirred-tank bioreactors constitute versatile workhorses well-suited for general-purpose uses requiring intense mixing and efficient mass transfer rates. Available in various sizes and materials, they can accommodate a wide array of cell lines and cultures.
4.2 Airlift Bioreactors: Gentle Mixing for Shear-Sensitive Cells
Airlift bioreactors present gentle alternatives for culturing shear-sensitive cells susceptible to damage induced by aggressive stirring mechanisms found in conventional designs. Instead, they rely on gas bubbles rising through draft tubes to induce circulation without imparting undue harm to vulnerable specimens.
4.3 Membrane Bioreactors: Combining Cultivation and Separation
Membrane bioreactors integrate separation membranes directly into the reactor setup, enabling continuous removal of secreted products and retention of live cells within the same chamber. Such arrangements streamline downstream processing steps and foster higher yields compared to traditional batch modes.
4.4 Packed Bed Bioreactors: High Cell Densities and Immobilized Enzymes
Packed bed bioreactors exploit solid supports packed tightly inside cylindrical columns to anchor adherent cells or immobilize enzymes securely. These setups permit operation at elevated cell densities and improved volumetric productivity, especially advantageous for slow-growing strains or resource-intensive metabolic transformations.
4.5 Fermenters: Tailored for Microbial Production
Fermenters epitomize dedicated hardware explicitly crafted to optimize microbial production rates by furnishing ample headspace for foam management, reinforced linings resistant to corrosion, and heavy-duty agitators primed for viscous broths teeming with industrious microbes.
Beyond the Vessel: Essential Lab Accessories and Consumables
No comprehensive discussion of bioreactors and fermentors would be complete without acknowledging vital ancillaries integral to successful operations:
5.1 Filtration Systems: Ensuring Product Purity and Sterility
Filtration plays a crucial role in any biomanufacturing process involving cell culture or fermentation. It serves multiple functions, ranging from removing particulate matter to concentrating valuable products and safeguarding sterility within the system. Various filtration techniques are available, each tailored to address specific challenges encountered during bioprocessing.
a) Depth Filters - These filters consist of layers of fibrous material arranged in depth configuration, capturing particles using mechanical interception, electrostatic attraction, or sieving effects. Common applications include clarification of feed streams, bioburden reduction, and particle removal preceding tangential flow filtration (TFF).
b) Surface Filters - Also known as membrane filters, surface filters possess thin porous sheets that retain particles upon encounter due to size exclusion properties. Ideal for microfiltration tasks where micrometer-sized particles must be separated from smaller molecules, they excel in applications such as sterilization, virus removal, and protein concentration.
c) Tangential Flow Filtration (TFF) - Unlike dead-end filtration methods, TFF employs crossflow forces perpendicular to filter elements, creating a dynamic equilibrium that minimizes fouling and prolongs service life. Beneficial for large-volume separations, diafiltration, and product recovery, TFF modules come in diverse formats compatible with skid-mounted installations or standalone setups.
d) Ultrafiltration (UF) / Nanofiltration (NF) - UF and NF membranes selectively separate molecules based on molecular weights, functioning as barriers for larger entities while permitting smaller ones to pass through freely. Often employed for buffer exchange, molecular weight cutoffs ranging from 1 to 100 kDa enable fractionation of proteins, nucleic acids, carbohydrates, and other macromolecular constituents.
5.2 Sensors and Monitoring Equipment: Maintaining Optimal Conditions
Precise regulation of physiochemical parameters forms the cornerstone of effective bioprocessing. Utilizing state-of-the-art sensors and monitoring instruments ensures accurate measurement and feedback control, ultimately leading to enhanced consistency and reproducibility. Here are some commonly measured variables and corresponding sensor types:
a) Temperature - Accurately controlling temperature is paramount for maintaining optimal cellular metabolism, enzyme activity, and reaction kinetics. Resistance temperature detectors (RTDs), thermistors, or thermocouples embedded within probes provide real-time temperature data, relaying information to automated controllers responsible for modulating heating/cooling systems.
b) pH - Since pH impacts biomolecule stability, ionic equilibria, and cell viability, maintaining near-neutral values becomes imperative during most bioprocesses. Glass electrodes coupled with reference junctions measure local hydrogen ion concentrations, transmitting signals interpreted by electronic meters displaying current pH values alongside historical trend graphs.
c) Dissolved Oxygen (DO) - Adequate DO levels are essential for respiring organisms reliant on oxidative phosphorylation for energy generation. Polarographic Clark-type electrodes or optical sensors quantitate DO tension, driving air/oxygen sparging or recirculation strategies aimed at preserving favorable redox balances.
d) Turbidity - Nephelometric or absorbance-based turbidity measurements gauge cell density indirectly, correlating light scattering patterns with increasing biomass accumulation. Online turbidostats leverage this relationship to automate feeding schedules, adding fresh medium proportionally to sustain exponential growth phases.
5.3 Bioprocess Media and Supplements: Fueling Growth and Production
Cells and microorganisms demand nourishment in the form of carbon sources, nitrogenous compounds, vitamins, minerals, trace elements, and growth factors to flourish and perform designated tasks effectively. Customizable basal media formulations containing defined mixtures of ingredients caters to diverse species-specific preferences, further augmented by judicious addition of supplements promoting robust health and heightened productivity.
a) Chemically Defined Media - Formulated exclusively from pure substances of known composition, chemically defined media eliminates uncertainties surrounding lot-to-lot variations inherent in animal serum-containing counterparts. Advantages include reduced risk of adventitious agent transmission, superior batch-to-batch consistency, and greater experimental rigor owing to precisely characterized components.
b) Serum-Supplemented Media - Certain applications necessitate inclusion of animal sera (like fetal bovine serum) to fulfill undefined growth factor requirements, support anchorage-dependent attachment, or stimulate quiescent cells entering dormancy. Despite drawbacks related to inconsistent compositions and ethical concerns, serum remains indispensable for particular scenarios despite advancements toward xeno-free alternatives.
c) Hydrolysates - Degraded polymers derived from natural origins (such as wheat germ, soybean, casein, or yeast extracts) release peptides, free amino acids, and low-molecular-weight sugars acting as alternative nutrition sources. Their utilization promotes sustainable sourcing, decreased dependence on animal derivatives, and provision of beneficial buffering capacities conducive to optimal cell fitness.
Partnering with IT Tech: Your One-Stop Shop for Bioreactor and Fermentor Solutions

At IT Tech, we pride ourselves on offering a broad spectrum of top-notch bioreactors and fermentors backed by expert consultation services, dependable maintenance programs, calibration expertise, and custom training sessions tailored to meet individual clientele demands. Explore our expansive inventory today and embark on your exciting journey towards unlocking novel discoveries and fostering groundbreaking innovations!
Products You may Like
Check out other IT- Tech product that suit your taste
Subscribe to our newsletter
Stay updated with IT-Tech Insights
Related posts
Check out other IT- Tech Scientific Resources
.png)
Integrating Bioreactors & Fermentors for Lab Efficiency
Discover expert tips and best practices for seamlessly integrating bioreactors and fermentors into your laboratory workflow. From selecting the right equipment to optimizing growth conditions, elevate your scientific endeavors with our comprehensive guide.
Transforming Scientific Discovery with Bioreactors and Fermentors
Explore the transformative potential of bioreactors and fermentors in driving cutting-edge research across biotechnology, pharmaceuticals, and more. Discover the latest advancements shaping the future of scientific innovation.
.png)
Making the Right Investment: A Detailed Analysis of Bioreactors
Discover the definitive guide to making informed investments in bioreactors. Explore the technical nuances, economic considerations, and supplier partnerships crucial for maximizing returns in bioprocessing. From single-use to stainless steel, gain insights to navigate the complexities and optimize your lab or production facility with expert advice and tailored solutions.










































































